CHICAGO--(BUSINESS WIRE)--Sep 25, 2023--
A peer-reviewed study in the Journal of the American College of Cardiology: Clinical Electrophysiology (JACC-EP) has found a significant reduction in the rate of atrioesophageal fistulas (AEFs) during cardiac ablation of the left atrium when using Attune Medical’s ensoETM proactive esophageal cooling device compared to luminal esophageal temperature monitoring. Esophageal injury can occur during ablation procedures for the treatment of atrial fibrillation, and an AEF is the most severe form of injury, resulting in death in most cases.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230925491005/en/
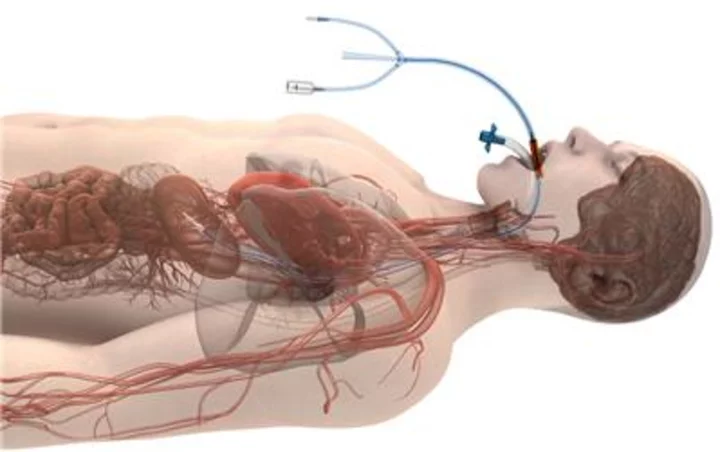
A peer-reviewed study in the Journal of the American College of Cardiology: Clinical Electrophysiology (JACC-EP) has found a significant reduction in the rate of atrioesophageal fistulas (AEFs) during cardiac ablation of the left atrium when using Attune Medical’s ensoETM proactive esophageal cooling device compared to luminal esophageal temperature monitoring. Attune Medical’s ensoETM is a single use thermal regulating device that is placed in the esophagus (similar to a standard orogastric tube) and connected to an external heat exchange unit, creating a closed-loop system for proactive controlled temperature management. (Graphic: Business Wire)
The study, Atrioesophageal Fistula Rates Before and After Adoption of Active Esophageal Cooling During Atrial Fibrillation Ablation, set out to compare the rate of AEFs before and after the adoption of proactive esophageal cooling. It incorporated data from over 25,000 patients in over 30 hospitals across the US and found:
- In 10,962 patients receiving luminal esophageal temperature monitoring, 16 developed an AEF.
- In 14,224 patients receiving the ensoETM to proactively cool the esophagus, none were found to have an AEF.
The authors concluded that adoption of active esophageal cooling during RF ablation of the left atrium for the treatment of atrial fibrillation was associated with a significant reduction in AEF rate.
“This is the first study of the effectiveness of any technique to show a significant benefit in the reduction of AEFs. The ensoETM device has proven to be remarkably effective at protecting our patients and it has become our standard of care,” commented first author Javier Sanchez, MD, FHRS, Cardiac Electrophysiologist at Texas Cardiac Arrhythmia in Dallas, Texas.
The ensoETM has been used to treat over 50,000 patients since first becoming available in 2015, and recently received De Novo marketing authorization from the US FDA to reduce the likelihood of ablation-related esophageal injury resulting from radiofrequency cardiac ablation procedures.
“Because the ensoETM is visible on intracardiac echocardiography, we’re able to perform our procedures without fluoroscopy, offering further benefits to patients and staff by reducing radiation exposure and eliminating the need to wear heavy lead protective shields throughout the case,” noted co-author Christopher Woods, MD, PhD, FHRS, Jim & Carol White Chair in Cardiovascular Research, Sutter Health.
Senior author Mark Metzl, MD, FHRS, Section Chief, Cardiac Electrophysiology at NorthShore University Health System, said, “I’d like to thank all our coauthors and collaborators for their work on this important study. In addition to the substantial safety benefits, we’ve found significant improvements in procedure time, outcome, and cost in the years since adopting ensoETM in our healthcare system, and this study further underscores the value of this technology.”
About ensoETM
Attune Medical’s ensoETM is a single use thermal regulating device that is placed in the esophagus (similar to a standard orogastric tube) and connected to an external heat exchange unit, creating a closed-loop system for proactive controlled temperature management.
About Attune Medical
Attune Medical’s novel technology platform pioneered the practice of using the esophageal space to proactively manage patient temperature and to reduce the likelihood of esophageal injury during cardiac ablation procedures.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230925491005/en/
Lisa Owens,lisammowens@gmail.com, 210.601.6647
KEYWORD: UNITED STATES NORTH AMERICA CANADA ILLINOIS
INDUSTRY KEYWORD: SCIENCE OTHER SCIENCE BIOTECHNOLOGY RESEARCH GENERAL HEALTH HEALTH CLINICAL TRIALS OTHER HEALTH
SOURCE: Attune Medical
Copyright Business Wire 2023.
PUB: 09/25/2023 10:00 AM/DISC: 09/25/2023 09:58 AM
http://www.businesswire.com/news/home/20230925491005/en