TOKYO--(BUSINESS WIRE)--Nov 1, 2023--
NIPPON KINZOKU CO., LTD. (TOKYO: 5491) (Headquarters: Minato-ku, Tokyo) commercialized “NK-304LCO” stainless steel for injection needles in November 2020, which is compliant with the Cobalt composition regulation of the European Medical Devices Regulation (MDR), which came into effect in May 2021 and will be fully transitioned on 31 December 2028.
We would like to announce that we are currently making progress in expanding sales to overseas markets.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231101657356/en/
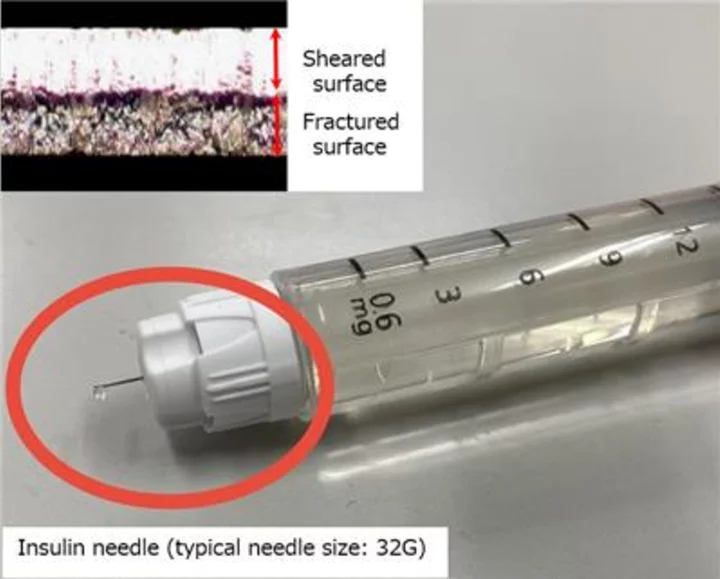
Stability of the welded part of the bare tube and cutting surface of the material, which will not rupture even when thinly drawn: The ratio between the fractured and sheared surfaces is agreed to facilitate welding by medical device manufacturers. The same end-face properties are supplied for the entire coil length (0.2 mm: approx. 4,000 M). (Graphic: Business Wire)
The MDR is a regulation for marketing medical devices in Europe and is a stricter approval system than the existing Medical Device Directive (MDD). The MDR stipulates the Regulation on Classification, Labeling and Packaging of substances and mixtures (CLP), which targets the cobalt (Co) component contained in stainless steel as a carcinogen. It is less than 0.1%.
Conventional NK-304NKM stainless steel for injection needles was difficult to adapt to this regulated quantity, but in response to requests from domestic and overseas medical device manufacturers, we have succeeded in alloy design (composition adjustment) as a result of discussions with raw material manufacturers. We were able to successfully supply the material.
Currently, we have started selling the amount equivalent to approximately 3% of the cobalt-regulated materials for customers using our stainless steel material for injection needles. The complete transition to MDR has been postponed from the original May 2024 to December 2028, volumes are currently increasing slightly due to the regulatory approach of individual medical manufacturers, but enquiries from the European and Chinese markets are increasing and sample shipments have already begun.
The NK-304NKM has been selected as the material of choice for "thin diameter" types of needles for insulin such as "painless needles" and cosmetic applications, and is currently being used all over the world. The sales share (*1) of our materials for injection needle applications is approximately 49% in Japan and 59% overseas (*2), with an average share of approximately 55%, and our quality superiority is widely recognized in the injection needle market. In addition to maintaining a high market share, sales to the Chinese and Indian markets are expected to expand in the future due to the increasing number of diabetes patients worldwide.
*1: 32 domestic and international companies, surveyed by us in October 2023.
*2: Overseas principal countries are South-East Asia, South Korea, China, India and the EU
Features of the NK-304LCO
Processability without rupture even at high drawing rate
Injection needles are manufactured by welding the edge of the material and then stretching the raw tube into a thin tube. For insulin, for example, a raw tube with a diameter of 4.0 mm and a wall thickness of 0.2 mm is drawn, then heat-treated and drawn many times in turn, until it is drawn to an outer diameter of 0.18 mm and a wall thickness of 0.05 mm. "Processability without rupture even when at high drawing rate" and "Stability of the welded part of the bare tube that does not fracture even when thinly drawn" are particularly important in this process.
Click here for the full text.
https://www.nipponkinzoku.co.jp/images/2023/10/91820e9f51d3ddb70d9857d1cacfef53-1.pdf
View source version on businesswire.com:https://www.businesswire.com/news/home/20231101657356/en/
CONTACT: Sales Development Dept.
NIPPON KINZOKU CO., LTD.
E-mail:nikkin-overseas@nipponkinzoku.co.jp
https://www.nipponkinzoku.co.jp/en/inquiry
KEYWORD: CHINA INDIA JAPAN SOUTHEAST ASIA ASIA PACIFIC EUROPE
INDUSTRY KEYWORD: MEDICAL SUPPLIES HARDWARE HEALTH STEEL DIABETES MEDICAL DEVICES TECHNOLOGY MANUFACTURING
SOURCE: NIPPON KINZOKU CO., LTD.
Copyright Business Wire 2023.
PUB: 11/01/2023 04:01 AM/DISC: 11/01/2023 04:03 AM
http://www.businesswire.com/news/home/20231101657356/en