PALO ALTO, Calif.--(BUSINESS WIRE)--Aug 22, 2023--
Recor Medical, Inc. (“Recor”) and its parent company, Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”) announce the U.S. Food and Drug Administration (FDA) Circulatory Systems Devices Panel of the Medical Devices Advisory Committee met to discuss the pre-market approval application (PMA) for the Paradise™ Ultrasound Renal Denervation (RDN) system, indicated to reduce blood pressure in patients with hypertension. The overall vote from the committee was favorable that there was sufficient data to support the use of the device in patients with uncontrolled hypertension.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230822990277/en/
(Graphic: Business Wire)
The committee voted 12 to 0 in favor of the Paradise Ultrasound RDN system with regard to safety and 8 to 3 in favor with regard to efficacy, with one vote abstaining. The committee also voted 10 to 2 in favor that the Paradise system benefits outweigh the risks.
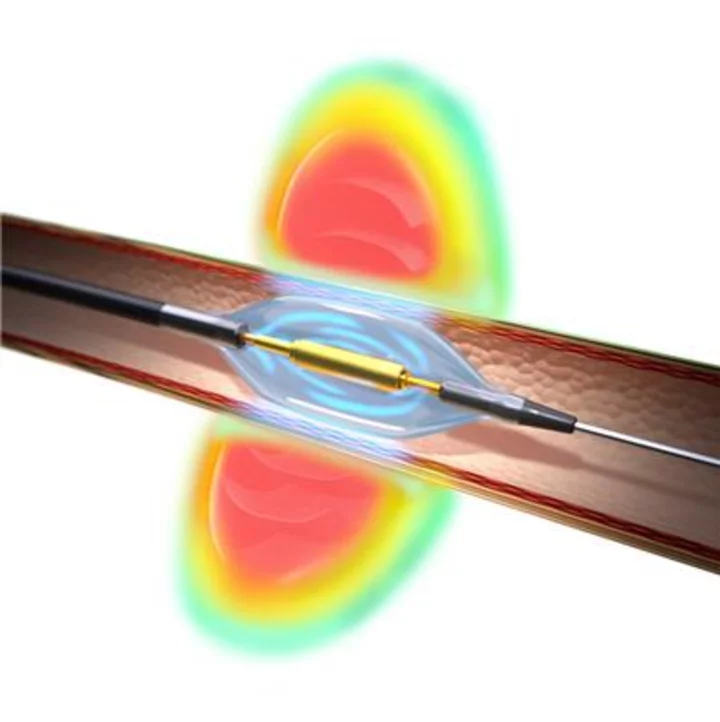
If approved by the FDA, the Paradise Ultrasound RDN system could be the first FDA-approved renal denervation device in the U.S. indicated to reduce blood pressure in patients with uncontrolled hypertension.
“Hypertension is one of the leading causes of cardiovascular morbidity and mortality, and despite the widespread availability of effective medications and lifestyle modification, rates of blood pressure control are still poor,” said Dr. Ajay Kirtane, Professor of Medicine at Columbia University, Vagelos College of Physicians and Surgeons / NewYork-Presbyterian Hospital, who serves as co-primary investigator in the RADIANCE Global Program studying the Paradise system. “I was pleased to see that the advisory committee recognized the adjunctive benefits of the Paradise Ultrasound RDN system in lowering blood pressure – something that we sought to demonstrate definitively through the generation of high-level scientific evidence within the RADIANCE Global Program.”
“We are thankful to the FDA and advisory committee members for the thoughtful review and discussion of the Paradise system for the treatment of uncontrolled hypertension,” said Lara Barghout, President and Chief Executive Officer, Recor Medical. “We will continue to work closely with the FDA in advance of our anticipated PMA approval and feel confident in the impact that Paradise Ultrasound RDN could have in addressing a significant unmet need.”
The PMA submission included data from three clinical trials in the company’s RADIANCE Global Program, all of which are independently powered, randomized sham-controlled clinical trials of the Paradise Ultrasound RDN system. The studies included more than 500 patients with mild-to-moderate or resistant uncontrolled hypertension.
The Paradise Ultrasound RDN system bears the CE mark for the treatment of hypertension and is an investigational device in the United States and Japan.
About Recor Medical, Inc.
Recor Medical, headquartered in Palo Alto, Calif., a wholly owned subsidiary of Otsuka Medical Devices Co., Ltd., is a medical technology company focused on transforming the management of hypertension. Recor has pioneered the use of the Paradise Ultrasound Renal Denervation system for the treatment of hypertension. The Paradise system is an investigational device in the United States and Japan and bears the CE mark. Recor has reported positive outcomes in three independent, randomized, sham-controlled studies of the Paradise system in patients with mild-to-moderate and resistant hypertension. In addition, Recor has begun the Global Paradise System (“GPS”) Registry in the European Union and the UK, with plans to expand globally.
About Otsuka Medical Devices Co., Ltd.
Otsuka Medical Devices focuses on the global development and commercialization of medical care products including endovascular devices that provide new therapeutic options in areas where patient needs cannot be met through pharmaceutical or other conventional treatment. Otsuka Medical Devices Co., Ltd. is a subsidiary of Otsuka Holdings Co., Ltd. ( www.otsuka.com/en ), a global healthcare company listed on the Tokyo Stock Exchange (JP 4578).
https://www.omd.otsuka.com/en/
View source version on businesswire.com:https://www.businesswire.com/news/home/20230822990277/en/
CONTACT: Annika Parrish
Health+Commerce
annika@healthandcommerce.com
KEYWORD: CALIFORNIA UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: BIOTECHNOLOGY FDA HEALTH MEDICAL DEVICES
SOURCE: Recor Medical
Copyright Business Wire 2023.
PUB: 08/22/2023 09:18 PM/DISC: 08/22/2023 09:18 PM
http://www.businesswire.com/news/home/20230822990277/en