Novo Nordisk A/S shares surged after a key study backed the use of Wegovy, its blockbuster weight-loss drug, to cut heart attacks and deaths in people with obesity and a history of heart disease.
The stock rose as much as 3.9% in Copenhagen after people taking the highest dose of Wegovy in the study saw a drop in blood sugar and inflammation, two harbingers of heart disease, alongside weight loss. That brought the shares’ gain to more than 52% this year, extending Novo’s run as Europe’s most valuable company.
Research is revealing a new frontier for drugs that are often thought of as an easy way to shed pounds; they’re potentially life-saving, too.
The results unveiled Saturday help cement the argument for using Novo’s drug as a heart treatment alongside statins and blood pressure therapies in high-risk people.
Heart disease is the No. 1 killer in the world, accounting for about a third of deaths. Novo plans to apply for approval for the drug to be used to reduce the risk of major cardiovascular events in overweight adults with established heart disease.
Eugene Yang, the chair of the American College of Cardiology’s prevention section, called the results “game-changing.” Doctors at the American Heart Association’s annual conference in Philadelphia erupted into several rounds of applause when the data were presented.
“Increasingly, physicians are understanding that this is not just about weight and appearance,” Lars Fruergaard Jorgensen, Novo’s chief executive officer, said in an interview on Friday. “It’s about real health benefits.”
Read More: All About the New Obesity Drugs Causing a Big Stir
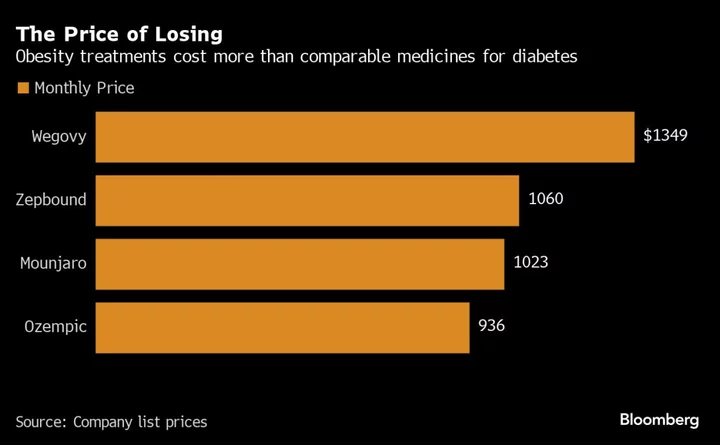
Weight-loss drugs such as Wegovy have become a phenomenon this year. Celebrities are touting the benefits, while investors handicap how much they will disrupt a wide swath of sectors that includes apparel companies, restaurants and packaged food producers. Novo is already struggling to keep up with demand. And competition is increasing with Eli Lilly & Co. just receiving approval for its weight-loss drug, Zepbound.
(Updates with year-to-date performance for shares in second paragraph)