TOKYO--(BUSINESS WIRE)--Oct 19, 2023--
Buzzreach Inc. (Headquartered in Minato-ku, Tokyo; CEO: Takateru Inokawa), a startup company, has become the first Japanese startup to join the Decentralized Trials & Research Alliance (DTRA), a Global organization dedicated to promoting decentralized clinical research.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231011227046/en/
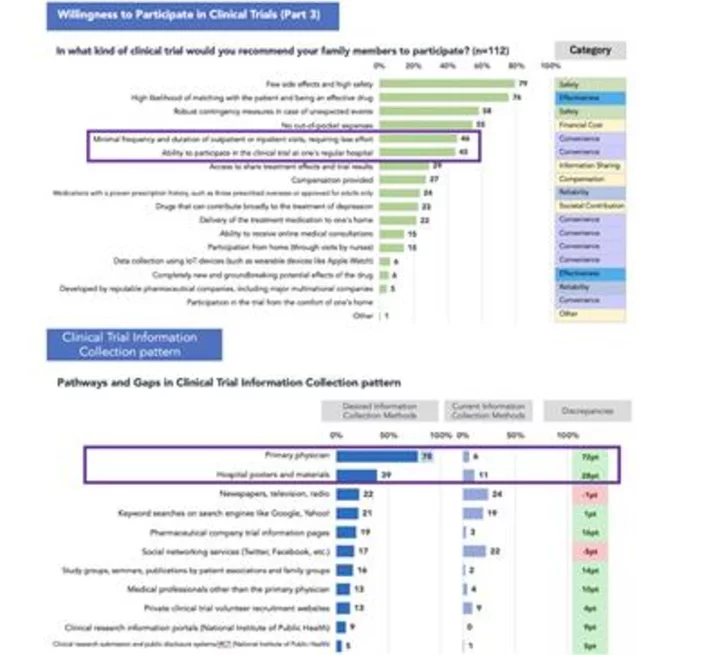
Willingness to Participate in Clinical Trials and Clinical Trial Information Collection pattern (Graphic: Business Wire)
Buzzreach is engaged in the development of a clinical trial and clinical research platform business that leverages the power of technology to address the challenges of a 10-year-long drug development process, aiming to shorten the time required for drug approvals. Additionally, they are involved in an SNS business connecting pharmaceutical companies and patients.
About DTRA
DTRA is an organization based in the United States that is composed of pharmaceutical companies, regulatory authorities, patient advocacy groups, clinical research organizations, and others. Its mission is to ease the global adoption of decentralized research methods. Through DTRA, stakeholders can collaborate to accelerate the adoption of patient-centric decentralized clinical trials and research in the life sciences and healthcare sectors.
About Buzzreach
Founded in June 2017, Buzzreach is the only vertical startup in Japan focused on solving challenges faced by pharmaceutical companies and investigative sites in the field of new drug development (clinical trials) using the power of technology. As the only vertical startup in Japan with a focus on clinical trials, Buzzreach aims to connect pharmaceutical companies, investigative sites, and patients from clinical development to the post-market phase, with the mission of "providing more options to as many patients as possible through the power of technology."
Buzzreach has raised approximately 1.8 billion JPY in funding to date, winning both the Best Award and Audience Award at G startup 1st batch hosted by Globis Capital Partners, one of Japan's leading venture capital firms. The company has also received accolades such as the Excellence Award at the Japan Healthcare Business Contest (JHeC) 2023 and inclusion in the Toyo Keizai "Great Venture 100."
Purpose of Joining DTRA
To create an efficient and patient-friendly environment for conducting clinical trials and specific clinical research, the adoption of decentralized clinical trials (DCT) is essential. However, the adoption rate of DCT in Japan remains low. According to a survey conducted by the Pharmaceutical Manufacturers' Association of Japan in August 2022, only 11.3% of companies had implemented decentralized clinical trials, while 52.8% responded that they had no plans to do so. There are still many challenges to overcome.
While there are numerous high-quality services for components of DCT such as eConsent, telemedicine, and decentralized modules conducted through home visits, there is currently a lack of comprehensive infrastructure to support DCT from the perspective of investigative sites and patients.
Based on a joint survey conducted by Buzzreach, BetaTrip, and PM-Link on awareness of clinical trials, in the survey of "what type of clinical trials they would recommend to their families," the module-based DCT environment ranked lower, and the only environment where they could participate in trials with their primary care physicians ranked higher in terms of convenience. Additionally, in the survey of "where they would prefer to obtain information about clinical trials," obtaining information from their existing primary care physicians or healthcare institutions with whom they have a pre-existing relationship ranked as a top choice.
As a result, it is believed that establishing an environment in which satellite sites, such as patients' regular physicians, can provide information on clinical trials and even handle certain trial procedures while considering Japan's cultural context will be a key point in promoting DCT in Japan.
With these considerations in mind, Buzzreach has joined DTRA to gain an understanding of the advanced DCT environment on a global scale and to contribute to the development of a DCT environment in Japan that aligns with the country's cultural nuances.
Participation in DTRA's 2023 Annual Meeting
Buzzreach will participate in the DTRA 2023 Annual Meeting in Boston from November 5th to 8th as the only Japanese company, where they will host a corporate seminar.
Comments from Takateru Inokawa, Co-founder CEO of Buzzreach
"As a specialized startup in the industry, we are honored to be the first to join DTRA. To promote the adoption of DCT in Japan, it will be crucial to determine to what extent our 'satellite sites,' which have a strong track record in patient recruitment, can be involved in essential aspects of clinical trials in the future. We will continue to make efforts to contribute to the efficient adoption and operation of DCT by stakeholders, while leveraging our expertise and incorporating the latest technology."
Company Overview
- Company Name: Buzzreach Inc.
- CEO: Takateru Inokawa
- Address: 3-19-1 Shirokanedai, Minato-ku, Tokyo 108-0071, Japan
- Founded: June 23, 2017
- Capital: 890,325,015 JPY (Including capital reserve)
- Website: https://www.buzzreach.co.jp/
Industry-First Platform Connecting Patients and Clinical Trial Institutions
Buzzreach offers various solutions to address challenges in clinical trials through its SaaS services:
- puzz: A SaaS service that solves various challenges in clinical trials and clinical research. Learn more
- Feasibility Concierge: A system for conducting feasibility assessments and selecting investigative sites. Learn more
- smt: A platform for clinical trial information disclosure and management. Learn more
- Study Works: A system for managing clinical trial operations and projects. Learn more
- Study Concierge: A clinical trial management app for patients (participants). Learn more
- VOICE: An app for collecting patient-subjective information. Learn more
- Miilike: A patient-focused social networking service. Learn more
View source version on businesswire.com:https://www.businesswire.com/news/home/20231011227046/en/
CONTACT: Contact Information
Buzzreach Inc.
Public Relations Contact: Ishizuka
Tel: +81-(0)3-4590-0258
Email:contact@buzzreach.co.jp
KEYWORD: UNITED STATES JAPAN NORTH AMERICA ASIA PACIFIC
INDUSTRY KEYWORD: START-UP TECHNOLOGY PROFESSIONAL SERVICES HEALTH GENERAL HEALTH PHARMACEUTICAL HEALTH TECHNOLOGY OTHER TECHNOLOGY SOFTWARE INTERNET CLINICAL TRIALS
SOURCE: Buzzreach Inc.
Copyright Business Wire 2023.
PUB: 10/19/2023 10:00 AM/DISC: 10/19/2023 10:02 AM
http://www.businesswire.com/news/home/20231011227046/en